By Dr Qingchun Yuan, Aston University. Qing is principal investigator of a CO2RE-funded project investigating diffuse methane removal and remediation by engineering chlorine radicals.
Methane, or CH4, is a powerful greenhouse gas. While its concentration in the air is 200 times lower than that of CO2, its warming potency is much higher. It has more than 80 times the warming power of CO2 over the first 20 years after it reaches the atmosphere [1].
In the pre-industrial time methane appeared in the air at approximately 715 parts per billion (ppb). Due to human activities, the concentration has increased to 1900 ppb as of 2021 [1]. The increase is responsible for a temperature rise of about 0.5°C, which accounts for nearly two-thirds of that caused by CO2 (~0.8 oC) so far [2].
“Methane is a powerful greenhouse gas… however, there is no technology available for the removal of diffuse methane.”
Methane is released naturally, for example, from decomposing organic matters of forest, and by human activities such as farming (especially animal husbandry), organic waste handling, coal mining and so on in diffuse forms. About 80% of the UK’s methane emissions come from such diffuse sources [3].
World methane budget studies show that the environment removes only part of the methane released. Without artificial removal of CH4 from the atmosphere, the methane concentration in the atmosphere will continuously increase and will cause an additional temperature rise of at least 0.2°C by 2050 [4]. Unlike the longer-lasting impact of CO2, CH4 can set the pace for warming in the near term. Taking action to reduce CH4 emissions will yield quick climate benefits that cannot be achieved by CO2 reductions alone. However, there is no technology available for the removal of diffuse methane (at a concentration of less than 2000 ppm).
One way the environment removes methane is by radicals. Radicals are molecules with an unpaired electron that makes them very reactive. Examples include hydroxyl radicals and chlorine radicals, which play a dominant role in oxidising methane into CO2 through a series of radical reactions. This accounts for more than 90% of the natural removal of methane.
Chlorine radicals initiate the oxidation 8-16 times faster than hydroxyl radicals, resulting in a significant acceleration of methane oxidation by up to 100 times [6]. I’d like to develop a feasible technology to engineer chlorine radicals and to safely remove diffuse methane, bridging the technology gap. The success of such an endeavour would have a wide range of benefits, positively impacting the climate, the economy of the UK, and global sustainability efforts.
Investigating the technology
Chlor-alkali process is the state-of-the art commercial technology for Cl2/NaOH production by electrolysing saturated brine, and Chlorine gas (Cl2) dissociates in the radiation of UV lights.
We will modify Chlor-alkali technology to effectively electrolyse seawater (use electricity to break down components of seawater into its individual elements), in order to generate chlorine gas. The chlorine gas generated will be subjected to a UV light to be split into chlorine radicals and initiate the radical oxidation of diffuse methane in the air. The development included:
- Investigating the electrolysis of seawater and the effect of brine concentration on Cl2 gas generation and concentration in the gas phase
- Investigating the effect of light wavelength and intensity on the photolysis (a chemical reaction where molecules are broken down or transformed when exposed to light energy) of Cl2 in the proposed environment (e.g. farms)
- Integrate the two lab operation units developed as a prototype device and test it for optimal operation conditions.
“We will effectively electrolyse seawater to generate chlorine gas. The chlorine gas will be subjected to a UV light to be split into chlorine radicals and initiate the radical oxidation of diffuse methane in the air.”
An electrolyser has been designed, installed, and tested for effective generation of Cl2 gas from seawater. A photochemical reactor has been designed, installed, and tested for Cl2 gas photolysis and reaction with methane (100 ppm) using different LED UV lights. The two operation units have been integrated and tested for the methane removal reactions. The experimental results have preliminarily approved the concept for the new technology development.
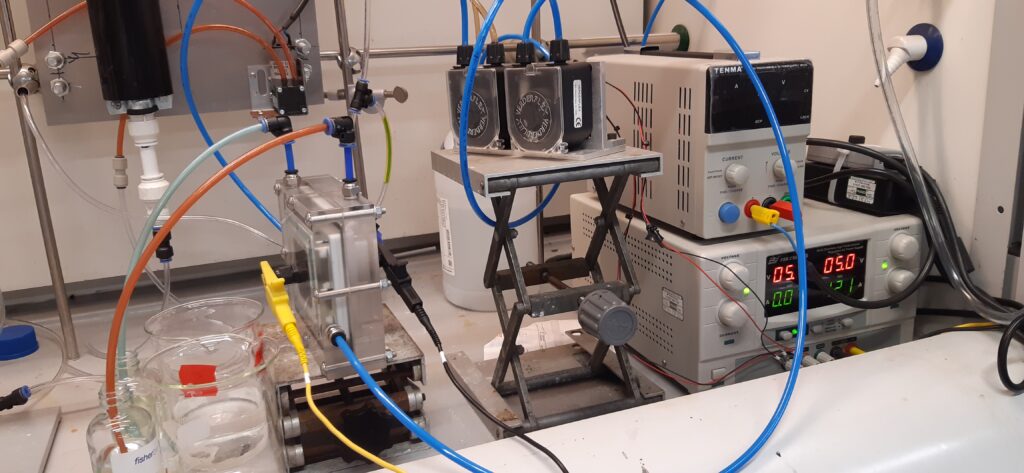
Next steps
More investigations are taking place, to:
- Design new photolysing reactors for more efficient photolysis of diffuse Chlorine molecules
- Improve the Chlorine gas capture system for desired concentration of Chlorine gas in a gas stream
- Improve the metering, detecting and control of chlorine gas from the seawater electrolysis in the moving gas phase online/inline
- Investigate the life cycle of chlorine for safe operations.
References
- NOAA (2021) https://gml.noaa.gov/ccgg/trends_ch4/
- IPCC, Climate Change 2021: The Physical Science Basis
- Department of Energy & Climate Change (UK), GHG Inventory Factsheets: Methane factsheet https://www.gov.uk/government/publications/greenhouse-gas-inventory-factsheets.
- https://ec.europa.eu/commission/presscorner/detail/en/statement_21_5206Saunois
- Hossaini, R. et al. Geophys. Res. Atmos. 2016, 121(23), 14271
- Finlayson-Pitts, B. Res. Chem. Intermed. 1993, 19(3), 235




